
Sample required:
Whole blood in a serum tube, minimum of 1ml per time point
Blood tube required:
Serum tube, red top or gold top
Diagnosis of hyperadrenocorticism (spontaneous or iatrogenic) and hypoadrenocorticism.
Treatment of hyperadrenocorticism.
Standard ACTH (aqueous solution) stimulation protocol
Vetnostics’ post-stimulation reference range and interpretation is based on this protocol. If using another protocol eg 1 ug/kg IV with testing exactly 60 minutes later (also adequate) or IM/SC protocols, the test details must be included. Synacthen can be stored frozen in appropriate aliquots for 6 months.
Depot ACTH (Synacthen® Depot) stimulation protocol
The Vetnostics reference intervals are not validated for this procedure. There is limited data available to assess the post-stim cortisol concentrations after depot Synacthen relative to the cortisol concentrations observed after aqueous Synacthen). From the limited literature available, testing at 60 minutes would appear to be the best approximation. Given that there is no longer a price advantage of depot Synacthen, we do not recommend this product routinely unless aqueous Synacthen is not available. There is no data that would validate freezing of any remaining depot Synacthen.
Following medical therapy for hyperadrenocorticism an ACTH stimulation test should be performed:
Trilostane
Perform the test 4-6 hours after administration of trilostane.
Mitotane
Perform the test 36-48 hours post administration of mitotane.
Sample required:
Whole blood in a serum tube, minimum of 4ml whole blood
Blood tube required:
Serum tube, red top or gold top

Sample required:
Whole blood in a serum tube, minimum of 0.5ml whole blood (minimum 200uL serum)
Blood tube required:
Serum tube, red top
For distinguishing between spayed and intact female dogs, cats and rabbits. This test may also detect females with ovarian remnant syndrome in an animal that was previously spayed.
For distinguishing between castrated and intact male dogs, cats, horses and rabbits. This test may also detect cryptorchid males.
For detection of granulosa cell tumour in mares.
Sample required:
Whole blood in a serum tube, minimum of 1ml whole blood per time point
Blood tube required:
Serum tube, red top or gold top
Sample required:
Bone marrow slides, Bone marrow aspirate in EDTA, Peripheral blood in EDTA
Blood tube required:
EDTA tubes for bone marrow and peripheral blood
Marrow sampling:
In dogs and cats, bone marrow can be aspirated using sterile technique from the iliac crest, femur, or humerus using an appropriately sized bone marrow aspiration needle and syringe. The site aspirated will vary according to several factors including size and age of animal and degree of obesity.
Smear preparation:
Bone marrow degenerates rapidly after collection. Smears should be prepared immediately after collection. Prepare as many smears as possible with the available marrow. Smears may be sent unstained to a laboratory, or may be stained using a routine haematological stain. Because bone marrow smears are thicker than ordinary blood smears, longer staining time or double-staining is required for adequate stain quality. Leave several smears unstained for possible future use for immunophenotyping or special stains.
Smear preparation with EDTA:A 2-3% EDTA:
A 2-3% EDTA solution (sterile) can be used to prevent clotting of the sample and to facilitate the preparation of smears. The syringe should be flushed with the EDTA solution, retaining no more than0.1 ml per 1.0 ml of marrow. The anticoagulated sample should be placed in a plastic Petri dish. The “spicules” or “unit particles” of bone marrow may be visible as glistening fat particles suspended in blood. Tilt the Petri dish so free blood flows to the side, leaving particles visible on the bottom of the plate. Using a microhaematocrit tube, carefully pick up several marrow particles using capillary action. Transfer the particles to a clean glass microscope slide and tap the tube gently to let them flow onto the centre of the slide. Place a second clean glass slide directly over the first (longitudinally), allowing the bone marrow to spread. Gently pull the top slide off the bottom slide, lengthwise, without exerting pressure on the slide. This should result in a central, oval-shaped monolayer of bone marrow cells surrounded by peripheral blood. The central area of the smear typically is rich in unit particles.
Smear preparation without EDTA:
If EDTA/isotonic saline solution is not used, as soon as a few drops of marrow sample appear in the syringe, the plunger is released, the syringe is detached from the needle, and the stylet is replaced in the needle. The needle remains embedded in the bone. The sample is immediately expelled directly onto a glass microscope slide that is tilted at 45-70º, allowing the sample to drain from the slide into a watch glass or Petri dish. Marrow flecks/particles tend to adhere to the glass microscope slide. Smears are then prepared as above.
A peripheral blood sample should also be obtained on the same day as marrow collection. This is essential because rapid changes can occur in peripheral blood counts and accurate interpretation of cells in the marrow require knowledge of FBC results.
If bone marrow cores are collected for histology in the same procedure, these must be shipped with a separate request form in a separate sample bag. Shipping cytology and histology samples to the laboratory in the same bag can result in exposure of the cytology samples to formalin, frequently making them unreadable.
Sample required:
Special ANTECH urine container containing fixative
Test description:
CADET® BRAF evaluates canine urine samples for the presence of cells containing a mutation for canine bladder/prostate cancer (Transitional cell carcinoma / Urothelial carcinoma). CADET® BRAF is a sensitive test designed to monitor the b-raf mutation in TCC/UC cases. CADET® BRAF testing can be used for both the non-invasive assessment of dogs displaying clinical signs consistent with TCC/UC and for confirmed cases undergoing treatment. The limit of detection of 10 mutation-bearing cells in a urine sample allows early diagnosis of a developing TCC/UC. Sensitivity of this assay is 85% and specificity is 99%.
CADET® BRAF-PLUS may additionally be indicated in dogs that present with clinical signs consistent with TCC/UC, but for which no BRAF mutation is detected. CADET® BRAF-PLUS detects more than 2/3 of the TCC/UC cases that are not identified by CADET® BRAF.
Clinical indications for CADET® BRAF and BRAF-PLUS
A specific ANTECH urine collection container must be used and can be ordered by calling 02 9005 7468.
40 mL of free catch urine (not cystocentesis or catheterised urine) is to be collected and placed immediately in the special urine container. The urine sample can be collected over 3 days, and once the urine is mixed with the preservative solution, the sample is stable at room temperature for several days, provided the sample is stored out of direct sunlight.
ANTECH paperwork is included with the urine container and must be completed and returned with the sample.
Test request:
On the Vetnostics General Request form, write BRAF (VBR) in the “Other test” area.
Leptospirosis is most common in younger dogs, of large breed and with outdoor access. Acute or subacute disease can occur with a wide range of clinical presentations.
In dogs with clinical signs and / or haematological and biochemical changes supportive of leptospirosis, two test modalities are available.
PCR
Dogs may be PCR positive before becoming serologically positive. Early in infection blood is the most sensitive sample. After 10-14 days, urine is the most sensitive sample. As the time of infection is rarely known, testing of both EDTA blood and urine concurrently is recommended.
Because of the biological behaviour of this organism, Leptospira PCR should be performed concurrently in both EDTA blood and urine to maximise the diagnostic outcome. PCR is more sensitive than MAT testing in the first 7-10 days of acute disease, due to the time period required for seroconversion.
In blood samples, sensitivity is highest in the first week at 84% then drops to 25% after the first week.
In urine samples, sensitivity has been reported as 69-100% depending on the time post infection.
When evaluating both EDTA and urine samples together, the positive predictive value was 100% and the negative predictive value was 74%.
Serology: MAT
Dogs become seropositive 2-3 weeks post infection. A single low titre is not diagnostic of infection, but paired titres at 3-week intervals can be evaluated. A single high titre is diagnostic.
Successful therapy results in a rapidly decreasing serological titre.
Sensitivity of a single MAT test has been reported as 22-67%. A single negative or low MAT titre in an acutely unwell animal does not exclude leptospiral disease.
When concurrent Leptospira PCR and MAT results are considered together,sensitivity is 84%
Further information can be found in the ACVIM Small Animal Consensus Statement on Leptospirosis.
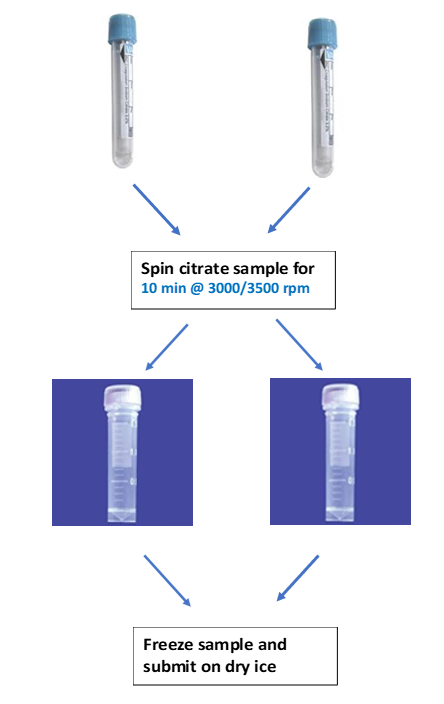
Sample required:
Citrate plasma, frozen, minimum of 2ml plasma.
Blood tube required:
Citrate tube
Animal Requirements:
An alternative test for von Willebrand’s is a genetic test which is available for a range of breeds with a known genetic risk. An EDTA blood sample is required for the genetic test, and samples can be sent directly to Orivet.
Sample required:
Correctly filled, in date citrate tube, EDTA sample (minimum 1 ml) if haematology is required, air dried blood smear
Blood tube required:
Citrate tube, EDTA tube
Effusion samples (peritoneal and Pleural effusions):
PCR detection of Feline Coronavirus in effusion fluids has a reported sensitivity of 72-100%. Several studies have reported a 100% specificity, but some studies have detected Feline Coronavirus in effusion fluids from cats with non FIP disease. This has been reported variably in 3-12% of control cats in these studies, usually with a low level of virus present.
The presence of Feline Coronavirus RNA, particularly in high levels, in an effusion that also has cytological and biochemical features suggestive of FIP, is highly supportive of a diagnosis of FIP.
Collection guideline:
Fluid in EDTA. Minimum volume of 0.5ml.
Mesenteric lymph node aspirates:
PCR for feline Coronavirus in mesenteric lymph nodes should only be performed when there is a strong index of suspicion for FIP based on clinical presentation and laboratory diagnostic tests. This should not be used as a screening assay in healthy cats.
A controlled study of Feline Coronavirus RNA detection in FNAs collected from the mesenteric lymph nodes from 20 cats with FIP without effusions reported a sensitivity of 90% and specificity of 96%.
Collection guideline:
Add 0.2 - 0.5 ml of saline into an EDTA tube.
Under ultrasound guidance, aspirate the mesenteric lymph node(s) using suction and add to the EDTA tube. Redraw the fluid from the EDTA tube though the needle and gently return into the EDTA tube. Repeat this process if necessary – the sample in the EDTA tube should be cloudy.
CSF:
Two studies have evaluated feline Coronavirus PCR on CSF samples and reported 100% specificity and a sensitivity of 30- 41.2%, however, not all cats included in these studies had neurological signs. When only cats with neurological and ophthalmological signs were considered the sensitivity of PCR was 86%. The same group found similar findings in a larger number of cats, where the sensitivity of PCR was only 30% when both neurological and non-neurological FIP cases were included but rose to 83.3% when only cats with neurological signs were included.
Collection guideline:
CSF in EDTA. Minimum volume of 0.2ml.
Cross matching of donor and recipient prior to transfusion.
Agglutination or haemolysis may interfere with results.
Canine: To differentiate between pituitary-dependent hyperadrenocorticism and adrenal-dependent hyperadrenocorticism in a dog confirmed to have hyperadrenocorticism (by LDDST or ACTH stimulation test).
Equine: To aid in the diagnosis of pituitary pars intermedia dysfunction (PPID; equine Cushing’s disease).
All equine abortions should be treated as infectious. Chlamydia psittaci has been documented in cases of equine abortion in Australia and is zoonotic. Use disposable gloves and outerwear which can be properly disinfected. A mask (P2/N95 or higher rating; surgical masks may not be effective) and goggles should be worn to protect the face from splashing fluids. For NSW submitters please provide PIC number (property identification code) on the request form.

Footnotes:
a = preferred sample is single dry swab of multiple sites across the placenta (amnion, umbilical cord between amnion and chorioallantois, chorioallantois near umbilical cord insertion). Alternative sample is fresh tissue (chorioallantois or pool of each of those sites).
b = amnion, umbilical cord between amnion and chorioallantois, chorioallantois near umbilical cord insertion, chorioallantois at cervical pole.

EHV-1 PCR, Chlamydia PCR (panel code VHT)
Summary
Fresh placenta, lung, liver, spleen, thymus for EHV-1 PCR, Chlamydia PCR
- separate labelled sterile screw top jars
- OR for placenta, single dry swab (multiple sites across placenta)
Fresh stomach contents, FNA needle cores of lung for Bacterial culture
- separate labelled sterile screw top jars (or swabs)
Formalin-fixed placenta, lung, liver, spleen, thymus for Histopathology- one screw top jar, if adequate formalin
Testing protocols for animal export are complicated and may change over time as countries update their requirements. It is the submitting veterinarian’s responsibility to ensure that all required testing is requested for the destination of the animal and that timing of testing is compliant with export country requirements.
To assist with some countries that are commonly exported to, we offer country-based protocols for New Zealand and South Africa.
The majority of export testing is performed at Vetnostics WA, with some testing performed at external laboratories.
Submission of samples
All samples and all request forms for export testing must be labelled with the animal’s name and microchip number. In addition to the routine Vetnostics request form, additional export submission forms must be completed for all testing performed at:
Tests offered include Trypanosoma smear evaluation, Babesia smear evaluation Ehrlichia serology, Leishmania serology, Microfilaria concentration, Heartworm antigen, Babesia gibsoni PCR, New Zealand and South Africa combination testing
For testing performed at Vetnostics WA:
Sample requirements including tube type and minimum volume for testing performed are included on the linked form.
For Brucella testing:
Sample required:
Whole blood in a serum tube, minimum of 2ml.
Blood tube required:
Serum tube, red top or gold top
Indications
1. For serological evaluation of dogs for export requirements only.
2. Serology for suspected clinical cases of Brucella cannot be accepted by Vetnostics.
Collection protocol
1. A serum sample is collected into a red top or gold top tube.
2. The microchip number must be written on the sample tube along with the animal and owner names.
3. An ACDP request form must be included with the sample, along with our routine Vetnostics request form.
For Rabies vaccination titre testing:
Sample required:
Whole blood in a serum tube, minimum of 2ml.
Blood tube required:
Serum tube, red top or gold top
Indications
1. For serological evaluation of cats and dogs for export requirements only.
Collection protocol
1. A serum sample is collected into a red top or gold top tube.
2. The microchip number must be written on the sample tube along with the animal and owner names.
3. An ACDP request form must be included with the sample, along with our routine Vetnostics request form.
For Leptospira serology
Sample required
Whole blood in a serum tube, minimum of 2ml.
Blood tube required
Serum tube, red top or gold top
Indications
For serological evaluation of dogs for diagnostic or export purposes
Collection protocol
1. A serum sample is collected into a red top or gold top tube.
2. The microchip number must be written on the sample tube along with the animal and owner names.
Comments
If the full panel is not required, it is the submitting veterinarian’s responsibility to specify which serovar(s) are required for testing (the requirement will differ from country to country).
If you have export testing requirements that are not included in the above information, please discuss testing requirements with a veterinary pathologist PRIOR TO SUBMISSION.
Ante mortem diagnosis of FIP remains a challenge. Clinical diagnosis of FIP should be based on a range of parameters including age, breed, number of cats in the household, clinical findings, haematology, biochemistry, analysis of effusions and specific tests for coronavirus or FIP.
A range of tests are available to evaluate cats with a clinical history and / or haematological and serum biochemical changes or effusion cytology supportive of FIP.
Histology
Histology is the gold standard diagnostic method. Typical pyogranulomas with vasculitis are diagnostic. Histology can successfully diagnose both effusive and dry forms of FIP with suitable sample selection. True cut biopsies have a lower diagnostic sensitivity than larger tissue samples.
Immunohistochemistry
Immunohistochemistry for Coronavirus can be used to confirm histological diagnoses, or to confirm the presence of FIP when non typical histological changes are identified.
PCR
Abdominal fluid:
PCR detection of Feline Coronavirus in effusion fluids has a reported sensitivity of 72-100%. Several studies have reported a 100% specificity, but some studies have detected Feline Coronavirus in effusion fluids from cats with non FIP disease.This has been reported variably in 3-12% of control cats in these studies,usually with a low level of virus present.
The presence of Feline Coronavirus RNA, particularly in high levels, in an effusion that also has cytological and biochemical features suggestive of FIP, is highly supportive of a diagnosis of FIP.
Abdominal lymph node aspirates:
PCR for feline Coronavirus in mesenteric lymph nodes should only be performed when there is a strong index of suspicion for FIP based on clinical presentation and laboratory diagnostic tests. This should not be used as a screening assay in healthy cats.
A controlled study of Feline Coronavirus RNA detection in FNAs collected from the mesenteric lymphnodes from 20 cats with FIP without effusions reported a sensitivity of 90% and specificity of 96%.
Collectionguideline:
Add 0.2 - 0.5 ml of saline into an EDTA tube.
Under ultrasound guidance, aspirate the mesenteric lymph node(s) using suction and add to the EDTA tube. Redraw the fluid from the EDTA tube though the needle and gently return into the EDTA tube. Repeat this process if necessary – the sample in the EDTA tube should be cloudy.
CSF:
Non-effusive FIP is associated with neurological signs in 25-33% of cases and ocular signs are common. Altered mental status, nystagmus, ataxia and seizures are reported. In cats with neurological FIP, CSF is often viscous and pleocytosis is identified in 67% of cases.
In samples from unwell cats (cats both with and without confirmed FIP), PCR of CSF had a sensitivity of 42% and a specificity of 100%. In cats with neurological or ocular signs, sensitivity was 86%.
Faeces:
Most Feline Coronavirus infections are subclinical. Self-limiting diarrhoea can occur on first infection although sometimes this can be severe. This is a small intestinal diarrhoea which usually resolves in 1-2 weeks but may become chronic. Chronic large bowel diarrhoea may occur in older cats who are Feline Coronavirus carriers. Post infection faecal shedding commonly occurs for 2-3 months but may be chronic, and some cats are lifelong shedders. Faecal shedding of Coronavirus is NOT diagnostic for FIP. Less than 10% of cats that have faecal shedding of Coronavirus will develop FIP.
Immunofluorescenceof effusion fluids
This assay detects direct immunofluorescent staining of FIP-related antigens in macrophages in effusion fluids. This test has high specificity (near 100%) but a moderate sensitivity of 75%.
Effusion fluids need to be collected into an EDTA tube, and must be submitted as fresh as possible,as these samples deteriorate rapidly.
Serological Tests:
Feline Coronavirus Qualitative Antibody (Immunocomb), Feline Coronavirus Quanitiative Antibody (IFA): These assays assess for coronavirus antibody in the blood. They are NOT specific for FIP.
They are relatively sensitive tests, however, can give false negatives with either very high viral loads or early in infection (within the first 3 weeks). Thus, a negative result does not exclude coronavirus infection or FIP. Positive results are consistent with exposure to Coronavirus but do not confirm FIP. Higher titres do not correlate with a higher risk of developing FIP, however cats with higher IFA titres are more likely to be shedding virus.
Peripheral Blood:
Effusion fluids:
Lymph node or organ aspirates:
Keep samples refrigerated until collection.
Sample required:
Urine
Blood tube required:
Sterile container
Investigation of suspected exposure or toxicity with common stimulants, depressants or strychnine.
Urine should be collected as soon as possible after presentation, if poisoning is suspected. Samples should bestored frozen if not immediately submitted to the laboratory.
The test panel includes the following compounds:

This test panel does NOT include 1080, organophosphates, metaldehyde or rodenticides. If poisoning with any of these agents is suspected, please refer to the Vetnostics Price List for additional information.
Sample required:
Spun gold top serum tube (see below), minimum 3ml
Blood tube required:
Serum tube, gold top
Evaluation of unexplained hypercalcaemia or hypocalcaemia.
Blood tube required:
Small red-top non-EDTA Sarstedt tube
Liver biopsy samples should preferably be greater than 100 mg in weight. Small tissue samples should be stored and transported in a suitable size container to minimise evaporative loss from the sample - ‘O’ ring sealed tubes are ideal for biopsy samples (small red-top non-EDTA Sarstedt tubes).Biopsy samples should be rinsed with saline and excess fluid/blood removed via gauze swabs prior to being placed into sample containers. The entire contents of the container will be assayed and is is not possible to differentiate tissue from blood/saline. All analysis and results for biopsies are on a wet weight basis.
Test code: TCV
Please request in Other/Unlisted Tests section.
Sample required:
Whole blood in a serum tube, minimum of 1ml per time point
Blood tube required:
Serum tube, red top or gold top
Diagnosis of hyperadrenocorticism
Sample required:
Frozen Serum minimum 1.0 ml
Blood tube required:
Serum tube, red top
Investigation of unexplained hypercalcaemia or hypocalcaemia.
When arranging a courier, advise a frozen collection is required.
Sample required:
Serum, 1ml
Blood tube required:
Red toptube (gold top gel tubes may interfere with drug levels)
Phenobarbitone:
After initiating drug therapy or after a change in dose rate, a steady state is achieved after approximately 2-3 weeks. After this time, samples for monitoring can be collected at a random time point. Trough sampling is not routinely required.
Drug monitoring is recommended at 2 weeks, 6 weeks and thereafter 6 monthly after starting medication or after dosage changes,
Potassium Bromide:
Potassium bromide has a very long half life of approximately 25 days. In general, measuring potassium bromide concentration every 6 weeks is recommended until the level is within the therapeutic range. Samples for monitoring can be collected at a random time point.
Digoxin:
A peak sample should be collected at 3-6 hours post the last dose.
Levetiracetam / Keppra:
Half life is estimated at 2-4 hours in dogs and 4-7 hours in cats. As such a steady state will not develop in dogs and will likely not develop in cats.
A fasting sample is required. A peak sample is collected 2-4 hours post therapy. Therapeutic range in dogs and cats is not defined. The human therapeutic range is 5-21 mcg/ml.
There are a range of diagnostic options for evaluation thyroid function in dogs and cats.
Thyroxine (Total T4)
Serum thyroxine is a common screening test in the evaluation of thyroid function.
Dogs
In dogs, total T4 (TT4) concentration within the reference interval usually excludes hypothyroidism. Rarely, increased TT4 is identified in dogs with clinical history strongly supporting hypothyroidism. In some breeds, this can be associated with anti-thyroglobulin antibodies. In such cases, cTSH and free T4 by equilibrium dialysis +/-evaluation of anti-thyroglobulin antibodies will usually allow diagnosis.
Dogs can have total T4 values below the reference interval with hypothyroidism, suppression by a range of medications (including corticosteroids and anti-convulsant therapy) or with euthyroid sick syndrome. Thus it is important to evaluate low, especially mildly low, total T4 results against the age, breed, clinical presentation, haematology and serum biochemistry results when considering if additional testing is indicated.
Serum thyroxine can also be tested in the rare situation of suspected canine hyperthyroidism. Increased TT4 is diagnostic for this unusual condition. Occasionally, normal dogs will have total T4 concentrations above the reference interval, especially younger animals.
Cats
In cats, an increased TT4 concentration is consistent with hyperthyroidism. Early in the disease, many cats have variable TT4, so high normal results should not exclude hyperthyroidism in a cat with supportive clinical signs. In such cases, repeating the TT4 in 2-4 weeks may be diagnostic.
Decreased TT4 in cats may be due to excessive response to therapy for hyperthyroidism, euthryoid sick syndrome or spontaneous hypothyroidism. The latter has formerly been regarded as rare but is being increasingly recognised in cats. Hypothyroidism in cats may have subtle presenting signs and is not necessarily accompanied by the haematologic or biochemical abnormalities seen in canine hypothyroidism.
Treatment
Total T4 is the assay of choice for monitoring therapy for hypothyroidism or hyperthyroidism. Samples from dogs on thyroid supplementation should be taken 6h after oral thyroxine administration, it is important to include the time post therapy that the sample was collected.
Canine TSH (cTSH)
Increased cTSH in a dog with suspected hypothyroidism supports the diagnosis. Approximately 70% of hypothyroid dogs will have an increased cTSH. TSH has also been used in the assessment of feline hypothyroidism and is also occasionally assessed in equivocal cases of feline hyperthyroidism.
Free T4 by Equilibrium Dialysis
Free T4 (fT4) by equilibrium dialysis is the gold standard for measurement of fT4 and Vetnostics do not recommend other fT4 assays. The sample must be sent overseas so there is a delay in result reporting. The combination of TT4, fT4 and cTSH is considered to be the gold standard for the diagnosis of canine hypothyroidism. Free T4 testing is also sometimes used in the diagnosis of hyperthyroidism (and hypothyroidism)in cats.
Toxoplasma gondii is a protozoan parasite that infects virtually all warm-blooded animals including humans. Domestic cats are the definitive host for the parasite but infection does not necessarily result from contact with cats or cat faeces. When cats become infected with toxoplasmosis they passunsporulated (non-infectious) oocysts (eggs) in faeces for 1 – 2 weeks. These non-infectious eggs mature in 1 – 5 days to become infectious (sporulated oocysts) and the sporulated oocysts survive for months to years. People can become infected by ingestion of these sporulated oocysts in contaminated soil or water. People can also become infected from ingesting the tissue cysts in meat. When animals (and people) become infected with toxoplasmosis, they may end up having tissue cysts in their body. Ingestion of poorly cooked meat (usually pork, goat or lamb) or failing to wash hands thoroughly after handling raw meat is probably the most common means of human infection with toxoplasmosis. The majority of people never realise that they have been infected as the signs are self limiting fever, enlarged lymph glands and malaise (just not feeling well). However, toxoplasmosis can cause serious disease in the unborn foetus, a major concern for pregnant women, and can also cause severe disease inimmunosuppressed people.. In immunosuppressed people, infection is usually due to a reactivation of tissue cysts in the person’s own body (eg from prior chronic infection not new infection). In pregnant women, infection of the foetus occurs after acute (new) infection. Stillbirths and serious foetal damage(especially eye and brain damage) can result. Touching cats is an extremely unlikely way to acquire toxoplasmosis and because of this, there is no correlation with toxoplasmosis and cat ownership.Similarly, veterinary health care providers are no more likely than the general population to be infected with toxoplasmosis, and people with HIV infection who own cats are no more likely to acquire toxoplasmosis than those who do not. There is one obvious conclusion from this:
THERE IS NO REASON TO REMOVE CATS FROM THE HOUSES AND LIVES OF PREGNANT WOMEN.
There is every reason to take sensible precautions:
NO CAT SHOULD EVER HAVE TO BE EUTHANASED OR REHOMEDDUE TO HUMAN PREGNANCY.
Sample required:
Fasting, nonhaemolysed sample. Whole blood in a serum tube, minimum of 2ml
Blood tube required:
Serum tube, red top or gold top
Sample required:
Urine, minimum 10ml, frozen
Blood tube required:
Sterile contained, frozen
For evaluation of adrenal masses where excessive catecholamine production is clinically suspected.
Sample required:
Serum, minimum of 1ml
Blood tube required:
Serum tube, red or gold top
Evaluation of vaccination status in dogs and cats.
Testing Available:
Dogs: Canine parvovirus, canine distemper, canine adenovirus.
Cats: Feline panleukopenia, feline herpesvirus, feline calicivirus.
Kennel cough serology is not available.
Samples can be collected at any time.
For further general information regarding sampling and tube requirements, please refer to our current pricelist.